Now Moves Back to the Cell Membrane Where It Fuses Once Again
These are notes from lecture iv of Harvard Extension'southward Cell Biology course.
The secretory pathwayrefers to the endoplasmic reticulum, Golgi apparatus and the vesicles that travel in between them also as the cell membrane and lysosomes. It'southward named 'secretory' for being the pathway by which the cell secretes proteins into the extracellular environs. Just equally usual, etymology only tells a fraction of the story. This pathway also processes proteins that will be membrane-bound (whether in the cellular membrane or in the ER or Golgi membranes themselves), every bit well every bit lysosomal enzymes, and too any proteins that will live their lives in the secretory pathway itself. It likewise does some things other than process proteins.
The cytosol and the 'lumen' (the liquid that fills the secretory pathway) are different chemical environments, and they ordinarily never mix. The cytosol is reductive (when you're in the cytosol, you keep meeting molecules that want to offer you electrons), and the ER, Golgi and extracellular environment are oxidative (molecules keep coming up to y'all request for electrons). See redox if notwithstanding confused. This makes for different protein-folding conditions: for instance, disulfide bonds usually only form in oxidative conditions. Moreover, unlike proteins may live only in the secretory pathway or simply in the cytosol. The secretory pathway provides a route for the cell to handle things that might not be good to have in the cytoplasm, and/or are almost useful when kept full-bodied in a specialized compartment with their desired interacting partners. Hepatocytes (in the liver) sequester drugs and toxins in the smooth ER and interruption them down for excretion from the torso in that location. The secretory pathway is not contiguous, merely every movement between its components is in little bubbled-off microcosms of its own chemical earth, chosen vesicles.
Many proteins that get through the secretory pathway never touch the cytosol – except the parts of membrane proteins that stick out on the cytosolic side. Many of them demand chaperones to help with folding, and/or a whole series of postal service-translational modifications in guild to be gear up for their native function, and the secretory pathway specializes in providing them all of that.
Today'southward lecture will focus on how proteins get translated into the ER and how they travel (in vesicles) between the ER, Golgi and other destinations. This is beautifully depicted in the Life of the Cell video:
The endoplasmic reticulumis the first step in the secretory pathway. Its membrane is continuous with the outer nuclear membrane, though information technology's non clear why that matters, since it's not like proteins begin their life in the nucleus. Rather, mRNAs drift around in the cytoplasm until they get picked upwards past a ribosome interested in translating them. In 'posttranslational translocation' the new poly peptide is moved into the ER after it'south translated. In the more than interesting phenomenon called 'cotranslational translocation' the ribosome starts translation just like whatsoever other protein, but somewhere in the first 16 to 30 amino acids information technology hits a signal peptide (aka signal sequence). That signal's motif is often 1 positively charged amino acid followed past 6-12 hydrophobic amino acids. This motif gets recognized by signal recognition particle (SRP, a 'ribonucleoprotein' or hybrid RNA/protein molecule) which binds to it and prevents the ribosome from continuing translation. Translation is stopped until the ribosome/SRP circuitous encounters an SRP receptor on the ER membrane. When they meet, SRP and its receptor each bind ane GTP molecule in the ER membrane, which manifestly strengthens their interaction. Fortuitously, this all happens adjacent to a Sec61 translocon – a protein complex that forms a aqueduct crossing the ER membrane. The translocon is actually a circuitous of three different proteins (genes: SEC61A1 or SEC61A2, SEC61B, SEC61G), of which the Sec61a subunit has x membrane-spanning a-helices which form the aqueduct. Once the ribosome is docked at the membrane it continues translation, pushing the point peptide and eventually the whole protein through the channel into the ER lumen. When translation stops, SRP and SRP receptor both hydrolzye their GTP to release each other and the ribosome cargo (this has to crave the energy of GTP, since the original binding was downhill), a point peptidase cleaves the bespeak peptide off of the nascent protein, and the protein is free to beginning folding in the ER.
A couple of other players are involved for some ER proteins. Oligosaccharide transferase, which adds glycosyl groups to asparagines in the nascent poly peptide, is part of the translocon complex and information technology really performs glycosylationwhilethe new protein is withal being translated. So although we phone call glycosylation a 'post-translational modification' it is actually made during translation in this case. Also, to achieve their proper structure, some proteins need to be fully translated earlier they are immune to start folding – if the N-terminal portion was allowed to start folding as shortly equally information technology entered the lumen, information technology would end up with the wrong overall structure. To prevent this, sometimes BiP the chaperone binds the poly peptide to proceed it unfolded for a while. Imagine BiP every bit another Pac-Man that bites downward on the protein to keep it linear, similar Hsc70 in the mitochondrial targeting process (see last week).
Hither'due south a video of it:
The first couple of minutes show the basic scenario described above. So information technology moves on to a more circuitous scenario I'll introduce in a infinitesimal. FYI, the video depicts two 'controversial' things not included in the in a higher place description: (1) the signal peptide existence degraded in the membrane, and (ii) a 'plug protein' that stops upwardly the channel before/after translation. Not all scientists hold on these 2 things however.
All of the proteins that nosotros know go through the secretory pathway were pinpointed there by people doing localization experiments to see where in the cell a poly peptide lies. A weird fact about the ER is that you can put the jail cell in a blender and afterwards the ER will just start reconnecting to itself, forming little 'microsomes' that are not attached to the nucleus simply form face-to-face bubbles of ER. You can then start to play games with proteases – which break down proteins – and detergents – which solubilize the ER membrane. Assuming your protein of involvement is translated, you can check if it (one) survives protease treatment but (2)doesn't survive protease + detergent treatment, then it'south a secretory pathway protein. The logic is that in case (ane) it was protected inside the ER, but in case (2) you dissolved the ER, so it got eaten by the protease. All this assumes you have an antibody or another way of detecting whether the poly peptide of interest is there afterward these treatments.
People also used such techniques to figure out that but 70 amino acids of a new poly peptide tin exist translated before it becomes too late for that protein to end up in the ER. Remember, the betoken peptide is in the first sixteen-xxx amino acids, and translocation to the ER depends on SRP beingness nowadays. Ribosomes translate at a predictable rate, so people got ribosomes started on translating some mRNA and then waited gear up amounts of time before adding SRP, to see how much translation could occur before SRP could no longer do its job.
The SRP receptor and the Sec61 proteins are ER membrane proteins – and there many other ER membrane, Golgi membrane and lysosome membrane proteins as well. In fact, even the membrane proteins (see form 02) of the jail cell membrane get candy in the secretory pathway. Many of these have several or tens of transmembrane domains (20-25 hydrophobic amino acids each) that have to be inserted in the correct guild and orientation (for instance, you lot really desire your ion channels and transporters pointed in the right direction, into vs. out of the prison cell). Accordingly there are a bunch of fancy biological mechanisms for getting these proteins inserted into the membrane correctly. This is what the latter half of the above video depicts.
So here's a tautology: some proteins take a topogenic sequence which determines their orientation in the membrane. This sequence is made of 2 types of signal sequences:
- a stop-transfer sequence (abbreviated STA for some reason) is a 22-25 hydrophobic amino acid sequence somewhere in the middle of the poly peptide that forms an alpha helix. When encountered it gets shoved into the membrane, and and so translation of the rest of the protein continues in the cytosol. And then this kind of 'undoes' the translocation to the ER that was started by the signal peptide at the beginning (North terminus) of the poly peptide.
- abespeak ballast sequence (abbreviated SA) is also a 22-25aa hydrophobic alpha helix, but with a series of ~3 positively charged amino acids on its left or right. Like the signal peptide, this is recognized past SRP, which brings the ribosome to the ER. Merely unlike the indicate peptide, this alpha helical sequence will be inserted into the ER membrane. The orientation of insertion is determined by the 3 positively charged amino acids. The positive charges take to always stop up on the cytosolic side, so if they come after (i.e. C-terminal of) the hydrophobic sequence, the protein ends up with its C final cease pointed into the cytosol, merely if they come before (i.eastward. N-terminal of) the hydrophobic sequence, the protein ends up with its Due north terminus pointed into the cytosol.
With those two signals as building blocks, you lot can imagine a protein with a series of stop transfer and indicate anchor sequences to create a whole series of back and forth transmembrane domains stitched into the membrane as if by a sewing auto. People have classified the membrane proteins into five categories:
- Blazon I has just a signal peptide and and then one finish transfer in the eye. Therefore it ends up with its (hydrophilic) N terminus in the lumen, its (hydrophobic) middle in the membrane and its (hydrophilic) C terminus in the cytosol.
- Type II does not start with a indicate peptide. Information technology starts out similar any other protein, but in the middle it has a betoken anchor sequence with the +++ amino acids coming offset and the hydrophobic series after. This makes the protein get translocated midway through translation, with the already-translated North-terminal function sticking out into the cytosol (since the +++ accept to stay cytosolic) and the now-beginninghoped-for-translated C-terminal part getting translated directly into the ER. So information technology ends upward transmembrane with its C terminus in the ER and N terminus in the cytosol – opposite of Type I.
- Type III is like Type II – no bespeak peptide, just a indicate anchor in the heart, but in this case the +++ come later on the hydrophobic sequence, which reverses the orientation. So this ends up with its Due north terminus in the ER and its C terminus in the cytosol. Opposite of Type II and, in the end, the same as Blazon I, though information technology got there in a different way – it does non have a signal peptide that gets cleaved off in the ER.
- Type Iv or 'multipass' proteins have an alternating series of signal sequences and stop transfer sequences. These are clearly more than than one 'type', yet are not nearly as various as your combinatoric imagination might allow. The orientation of the first betoken sequence determines whether the N terminus volition end up in the cytosol or ER, and total number of stop transfer + signal ballast sequences determines where the C terminus will terminate up: an fifty-fifty number = same side equally N terminus, odd number = reverse side every bit Due north terminus. The STA and SA sequences take to strictly alternate, with the exception that you can start with 2 signal ballast sequences if the first one is oriented with the N terminus into the cytosol. Just to brand a mockery of this categorization scheme, people have divers some incompletely-defined subtypes of Type IV, where Type IVa is N-terminal in cytosol (thus it starts like a Type II poly peptide) and Blazon IVb is Due north-terminal in the lumen (it starts like a Type Iii protein but and then has some other SA sequence that puts it back into the ER). GLUT1 from Course 02 is a Type IVa.
- GPI-anchored proteins, which are the fifth type just aren't called Blazon Five, start with a indicate peptide and end with a hydrophobic C-terminus which stays embedded in the membrane. That hydrophobic end gets cleaved off and replaced with GPI, which also stays embedded in the membrane. PrP is one of these – more on that after.
By at present nosotros've discussed how proteins can end upwardly in the ER lumen or spanning the ER membrane. Virtually proteins leave the ER within minutes, transported in vesicles leap for the Golgi and then after for excretion, lysosomes or the cell membrane. That forward direction of travel is called anterograde; going backwards from Golgi to ER is retrograde transport.
Both types of send accept place in membrane-bound vesicles. These bud off of the membrane of wherever they're coming from, and later fuse to the membrane of wherever they're headed – beautifully depicted at ~2:25 in the Life of the Prison cell video higher up. The body from which the vesicles form is the 'donor compartment', and the destination they after fuse to is the 'acceptor compartment'.
The budding procedure requires that G proteins in the membrane recruit Glaze proteins. Specifically, for anterograde send, Grand protein Sar1 (cistron: SAR1A) recruits COPII ('cop two'); for retrograde transport, an ARF 1000 protein recruits COPI (pronounced 'cop i'). These G proteins are activated to do this job when Gef loads them with GTP, swapping out GDP.
So the steps in anterograde send, for example, are equally follows:
- Sec12-GEF (Sec stands for secretory) loads Sar1 with GTP. When bound to GDP, Sar1 but floats around the donor compartment, only when leap to GTP, it undergoes conformational change that causes its otherwise-cached N-final hydrophobic tail to protrude, making it stick into the membrane, where COPII proteins and then start to accumulate considering they really like that tail.
- The COPIIs beginning to polymerize and, due to its conformation, have an intrinsic preference for curvature, so their accumulation starts to make budding happen. At the same fourth dimension, membrane bound proteins that need to be transported – identified past a DXE (i.e. aspartate-anything-glutamate) amino acrid sequence that forms a binding site in their cytosolic part – become recruited to the newly forming vesicle. Membrane-bound proteins human activity equally receptors, recruiting lumenal proteins that are jump for the Golgi to hang out in the concave infinite where they'll end up in the vesicle one time it forms.
- Once enough COPII take arrived, the vesicle buds off, at which betoken Sar1 hydrolyzes its GTP, providing the energy for it to suck its hydrophobic tail back into itself, cutting the COPIIs loose. The vesicle is now disconnected from the donor compartment.
- Now, for poorly explained (or poorly understood?) reasons, the glaze of COPIIs just disassembles, exposing receptors under the coat which direct the targeting of the vesicle. Once the vesicle arrives at its destination, Rab-GTP embedded in the vesicle membrane interacts with a Rab effector embedded in the acceptor compartment membrane. A sideways glance is exchanged, involvement is kindled. Soon the vesicle will fuse to the membrane.
- SNARE proteins nowadays on both the vesicle and target membrane (V-SNARE and T-SNARE respectively) interact to bring the membranes even closer. In this example we'll consider VAMP (the VAMP_ genes) as the V-SNARE and Syntaxin (the STX__ genes) and SNAP25 (SNAP25 cistron) every bit the T-SNAREs. Syntaxin and SNAP25 are both membrane proteins; Syntaxin has 1 blastoff helix and SNAP25 has 2, all on the cytosolic side. The alpha helices drive the interaction with VAMP. The opposing sides' alpha helices have extremely strong affinity for one another, bringing the membranes shut enough to fuse. One time this has happened, prying the 5-SNAREs and T-SNAREs apart once more requires two proteins: NSF (gene: NSF; stands for NEM sensitive factor) and alpha-SNAP (factor: NAPA), a soluble NSF attachment protein. NSF is an ATPase, and burns ATP to drive the energetically uphill disassembly of the complex.
Now for retrograde transport. Why is there retrograde ship at all? Here is a non-exhaustive list of some reasons:
- Some membrane proteins start their life in the ER, demand to get modified in the Golgi, but and so demand to get dorsum to the ER. They practice this with a KKXX amino acid sequence.
- There's also a KDEL amino acid sequence at the C terminus of some lumenal proteins which is suppsoed to continue them in the ER, merely it'south not perfect – sometimes they cease up in the Golgi, in which case they're targeted dorsum to the ER via retrograde transport dependent on that KDEL sequence for recognition. The mechanism is kind of dandy – the proteins that recognize and bind to KDEL do and then only at low pH, and the pH of the Golgi is lower than the ER, so they bind KDEL in the Golgi, then release information technology when they're back in the more neutral pH of the ER.
- Also, think about it, all the proteins that participate in anterograde transport – the V-SNARES, Rab, etc. – have to get dorsum to the ER so they can do it all over once more, like how the bus has to go dorsum to the bus depot at the end of the solar day.
- Equally we'll see shortly, the Golgi come in multiple stages which depend on the addition of enzymes from further downstream.
The process of retrograde transport is not then dissimilar from anterograde. It uses ARF instead of Sar1, COPI instead of COPII, but information technology works the same: ARF loaded with GTP lets its hydrophobic tail stick into the membrane, alluring the attending of COPIs. COPI has 2 components, COPIalpha and COPIbeta, both of which collaborate with that KKXXX sequence to recruit membrane-bound proteins destined for retrograde transport. Some proteins besides accept an RR sequence (anywhere in the protein) which can flag them for retrograde ship.
The Golgi appliance is not contiguous. It is a stacked set up of separate subcompartments called sacs or cisternae. Different compartments have dissimilar properties and proteins visit them in a particular lodge. In order from ER to prison cell membrane, the Golgi compartments are called cis, medial, trans and trans-Golgi network. Each compartment has different enzymes that modify proteins, and the modifications have to happen in a certain order, hence the demand for a stacked set of compartments.
But as proteins mature in the Golgi, it'due south not every bit though they bud off in vesicles from one compartment and move to the side by side. Rather, the compartment they are already in moves outward and 'matures' equally new enzymes are added to information technology (from further downward the Golgi concatenation) via retrograde send. Weird, correct? It's kind of similar if instead of moving from an uncomplicated school to a middle schoolhouse to a high school you just stayed in one school building for your whole childhood and boyhood, and they just brought in new textbooks and teachers every year to continue it advisable to the grade that you and your classmates had now reached. Hither'south what the Golgi look like as they movement and evolve:
So at that place's (trivial or) no anterograde transport within the Golgi, but plenty of retrograde transport to bring each new round of enzymes in. When proteins have finally completed the full M-12 curriculum of the Golgi network, they do undergo transport to movement on to their concluding destinaton. They bud off in a vesicle which volition get one of three places:
- Exocytosis – fusion with the cell membrane. Thus the lumenal proteins volition exist secreted extracellularly, and the membrane proteins volition go jail cell membrane proteins.
- Secretory vesicles – these just stick around every bit vesicles in the cell until needed – where 'needed' means they do somewhen undergo exocytosis. In neurons, this is where neurotransmitters are stored until an action potential demands their secretion into the synapse. In the breadbasket, the cells that produce gastric enzymes keep those enzymes in secretory vesicles until nutrient intake triggers their release into the stomach.
- Lysosomes - where misfolded proteins go to get degraded.
The send from the trans-Golgi network on to these destinations is different from the other transport discussed above and often involves clathrin (CLT__ genes). Vesicles budding off take a two-layer glaze, with adapter protein (AP) complexes as the inner layer and clathrin as the outer layer. The adapter proteins have a target signal with a YXXh motif (h = Φ = whatsoever hydrophobic amino acid). Clathrin forms the and so-called 'clathrin-triskelion' formation shown here:
(Image thanks to Wikimedia Eatables user Phoebus87)
Clathrin is also responsible for endocytosis – budding off of vesicles of extracellular stuff (and prison cell membrane proteins) to comeintothe cell. This is called clathrin-mediated endocytosis. Receptors in the jail cell membrane get endocytosed very frequently: the whole population of hormone receptors turns over about every hr, particularly when hormones are being received. Taking upwardly the receptor into a vesicle is one manner for the jail cell to cut off the incoming signal until information technology can be processed.
The plasma membrane notes discuss cystic fibrosis briefly: CFTR is an ABC transporter responsible for pumping Cl- out of the jail cell (information technology besides lets Na+ in). Loss-of-function mutants don't pump Cl-, which removes the driving force for osmosis, thickening the mucus and causing animate problems. There are at least 127 different loss-of-function CFTR mutants (at least, that'southward how many Natera tests for) that (if both alleles are disabled) cause cystic fibrosis. The nearly common mutation is ΔF508, which is ~three% of all European CFTR alleles and almost 70% of mutant ones. The loss of that i phenylalanine changes CFTR's conformation and so that the di-acidic leave code (amino acids D565 and D567) that targets CFTR for exocytotic vesicles is no longer correctly exposed and the protein never makes it to the cell membrane [Wang 2004].
discussion section
In section we read Hu 2009, who showed that atlastin proteins are involved in creating the tubular ER network. The evidence came most entirely from protein-protein interactions. I was surprised this newspaper was a big deal, because there have been a 1000000 papers showing poly peptide-protein interactions for huntingtin, and no one really believes all of them and it hasn't necessarily gotten us any closer to knowing what huntingtin does or what goes wrong in Huntington's Disease. But obviously Hu was able to brand a pretty clean case for the atlastins' interactions with reticulons as implying a role in ER formation. It helps that Hu was able to prove a 'genetic interaction' in addition to a physical (binding) interaction. A 'genetic interaction' (I had to look information technology up) means when "Sometimes mutations in ii genes produce a phenotype that is surprising in light of each mutation's individual effects. This phenomenon, which defines genetic interaction, tin can reveal functional relationships betwixt genes and pathways." [Mani 2007].
PrP
This is a decade old, so some stuff may be outdated, but I establish Harris 2003 (ft)'s review of PrP cell biology extremely clear and helpful. Kim & Hegde 2002 was also helpful. PrP is a secretory pathway protein. Its starting time 22 amino acids (MANLGCWMLVLFVATWSDLGLC) are a signal peptide that causes cotranslational translocation to the ER. Normally, PrP only gets GPI-linked at its C terminus and is anchored to the exoplasmic side of the membrane. Just amino acids 111-134 (HMAGAAAAGAVVGGLGGYMLGSAM) are a sort of weakpoint anchor sequence (Type 2, with the +++ amino acids coming before the signal anchor) thatsometimes only not ever becomes a transmembrane domain, inverting the C terminus into the lumen. Fifty-fifty more than confusingly, that sequence can sometimes just end upwardly equally a transmembrane domainwithout the inversion, so that the N terminus is in the lumen. So there are three membrane topologies of PrP: regular quondam GPI-anchored, and two transmembrane orientations, equally depicted in Harris 2003 Fig 3:
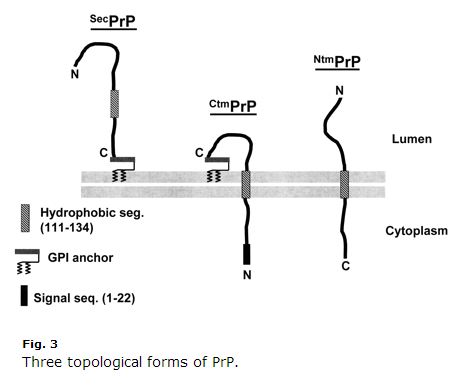
Annotation how weird CtmPrP is. It'south transmembrane yet too GPI-anchored, and the N-final bespeak peptide is never broken off. Usually, the transmembrane forms are < 10% of total PrP. In some laboratory weather the per centum is higher, and two of the GSS-causing mutations (A117V and P105L) likewise increase the fraction of CtmPrP to 20-30% of all PrP. Of these three forms, there is a good corporeality of testify that CtmPrP is toxic, and that information technology might play a function in prion formation, though well-nigh genetic prion affliction mutations (including FFI D178N) do non appear to affect the membrane topology of PrP or the fraction ofCtmPrP.
After PrP goes through the Golgi, it is targeted for the cell membrane. But according to Harris, it doesn't just sit down at that place – it frequently through clathrin-mediated endocytosis and cycles through the cell every ~60 minutes, with some molecules being cleaved on each wheel. Copper stimulates this endocytosis of PrP. Most genetic prion disease mutations change the localization of PrP – usually when a mutation is present, less PrP is found on the cell surface, with more accumulating in the ER.
Source: https://www.cureffi.org/2013/02/24/cell-biology-04-the-secretory-pathway/
0 Response to "Now Moves Back to the Cell Membrane Where It Fuses Once Again"
Enregistrer un commentaire